Innovation
Innovation examples
Innovation
Stem cell derived Vessels-on-Chip to study brain disorders
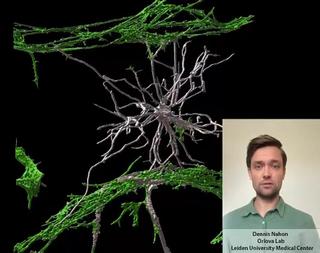
Dennis Nahon is a PhD candidate in the Department of Anatomy and Embryology at the Leiden University Medical Center. In his research, under supervision of Dr. Valeria Orlova (https://www.orlovalab.com/) and Prof. Dr. Christine Mummery, he aims to mimic a blood vessel in the brain by combining different stem cell derived cell types, in a 3D Vessel-on-Chip model. Here, an example of these in vitro blood vessels is shown in which certain brain cells known as astrocytes (in white) interact with the blood vessels (in red). This model paves the way for investigating brain vessels outside the human body, while reducing the need for animal models.

Innovation examples
Innovation
From 2D hiPSC culture to developing a 3D vessel-on-chip
Theano Tsikari is a 2nd year PhD student at the Orlova group at LUMC. As part of the LymphChip consortium, her project focuses on the development of immunocompetent organ-on-chip models of the cardiovascular system, and especially the integration of tissue-resident macrophages and lymphatic vasculature using human induced pluripotent stem cells. In this video, you can follow her as she presents you the backbone of her project, a 3D hiPSC-derived vessel-on-chip model, that has been previously developed in the Orlova group and can be employed for the generation of advanced in vitro models of vascular diseases.

Innovation examples
Innovation
Unified organoid system for modeling heart and kidney interaction on-a-chip
Beatrice Gabbin is a PhD candidate at the Anatomy and Embryology Department of the Leiden University Medical Center. Her project is shared with the Nephrology Department and focusses on the study of the cardiorenal axis in vitro. Both heart and kidneys have vital functions in the human body and reciprocally influence each other’s behavior: pathological changes in one can damage the other. There are already multiple independent in vitro (human) models of heart and kidney, but none have so far captured their dynamic crosstalk. The aim of the project is therefore to develop a microfluidic system which can be used to study heart and kidney interaction in vitro. For this purpose, cardiac microtissues and kidney organoids derived from human induced pluripotent stem cells are generated and loaded onto a 3D perfusion chip for their dynamic co-culture. This system enables the study the cardiac and kidney interaction with a high level of control. The validation of a unified organoid system will enable the investigation of diseases involving the two organs and their potential treatments. Read more via the link in the video and https://doi.org/10.1016/j.mtbio.2023.100818.

Innovation examples
Innovation
Modelling COVID-19-induced thrombosis using blood-perfused Vessels-on-Chips
A subset of hospitalized COVID-19 patients develops severe symptoms like microthrombosis and multiple organ-failure, worsening survival rates. The most inner layer of cells of a blood vessel, the endothelial cells, play a central role in the development of these complications. Their dysfunction can be replicated in advanced cell culture models like our blood-perfused Vessel-on-Chip to further understand disease mechanisms. In this short highlight, Huub Weeber from the University of Twente shows how the technique works and what these models contribute to our knowledge of COVID-19.

Innovation examples
HealthToxicologyInnovationIn vitro
Development of 3D liver spheroids
Human-based in vitro models are increasingly being used in the hepatology field. And in addition to the obvious ethical arguments, they offer several advantages over the classical animal models. One of them is the ability to perform mechanistic research at the molecular level in a well-controlled setting and reduce species differences. These liver-based in vitro models can range from simple monolayer cultures of hepatocytes to the liver-on-chips systems in which all liver cells are cultured in a 3D configuration on a microfluidic platform. Liver-based in vitro models must be selected on a case-by-case basis and should fit the purpose of the research, which might go from fundamental to translational research.

Innovation examples
HealthToxicologyInnovationIn vitro
Platform for in vitro airborne inhalation testing
The air-liquid interface (ALI) technique uses lung cells cultured on a tiny polymer membrane in a cup. On one side of the membrane is a liquid containing the medium necessary for the cells to survive, while the other side is in contact with air. This is similar to the situation in the human lung. The compound to be tested is administered via an aerosol, vapor, or gas to mimic the situation in human lungs. By monitoring different parameters in the cell model before and after the compound is added, it is possible to measure the effects on lung cells. Depending on the test to be carried out, the lung cells can come from different regions in the respiratory tract and even from a variety of people, including individuals who smoke a lot or have specific diseases such as chronic obstructive pulmonary disease or asthma.
In vitro ALI inhalation testing (https://doi.org/10.1021/acs.est.7b00493) adds value for e.g. pre-clinical trials and research in the pharmaceutical industry and testing (new) compounds for the chemical sector and beyond. The advantages of ALI inhalation testing are that it is a non-animal method, it reduces the use of in vivo experiments, pre-clinical testing with human-derived cell models is more realistic and limits clinical trial failures and it provides faster and more efficient testing of compound

Innovation examples
HealthInnovationIn vitro
Using skin and mucosa models to replace animal testing
The skin and mucosa are important tissues that differ between species in health and disease. The group of Sue Gibbs works on the development of advanced in vitro models that mimic these two tissues, specialising in immunity models and organ-on-a-chip technologies. They use skin models to study for example melanoma, skin allergies, eczema, burns and healing wounds. Dental models are used for the safety of materials used in dentistry, for example to test the quality of the implant and false tooth when it comes to attaching to the soft tissue. Their ambition is to expand into the field of multi-organ technology to make even more relevant models for the human skin and mucosa.
Click on the link in the video to watch more or read the interview with Sue he[https://vu.nl/en/research/more-about/using-skin-and-mucosa-models-to-replace-animal-testing]re.

Innovation examples
HealthInnovationData
Using data and computational modelling in biomedical research
Bioinformatics and systems biology hold great promise to translate the wealth of biological data into meaningful knowledge about human health and disease. The group of Bas Teusink helps biologists to deal with high throughput data, for example metabolomics (how cell metabolism works) and proteomics (how protein networks work) from patient material or cell cultures. This can help to better understand disease mechanisms and aid drug targeting or personalised medicine. In the future, combining data from different models (in vitro, in vivo and human data) could become a digital model of humans, or a “ digital twin”.
Click on the link in the video to watch more or read the interview with Bas (and Jaap Heringa) he[https://vu.nl/en/research/more-about/using-data-and-computational-modelling-in-biomedical-research]re.

Innovation examples
HealthInnovationIn vitro
Treating genetic heart disease using engineered heart tissue
Some heart disease are caused by a gene mutation in the cardiac muscle cells. People with this genetic disease are affected it between the ages of 20 and 40, and there is no preventative treatment for this. The group of Jolanda van der Velden works on the development of engineered heart tissue made from human stem cells to unravel disease mechanisms and test drugs to treat the disease. They use different kinds of stem-cell-based cultures. 2D cell cultures are useful to test a large number of candidate drugs, while patient-derived stem cells that are differentiated in heart cells can help to get detailed understanding of the disease and test the most promising treatments.
Click on the link in the video to watch more or read the interview with Jolanda here (https://vu.nl/en/research/more-about/treating-genetic-heart-disease-using-engineered-heart-tissue).

Innovation examples
HealthInnovationIn vitro
Using human organoid technology to treat viral infections in children
Viral infection in (very young) children can be detrimental to their neurological health. The mechanisms of some viruses work very differently in children compared with adults, which is not well understood yet. The research group of Dasja Pajkrt studies viral infections in children from the clinic by using human-derived organoids. They focus on three groups of viruses that can severely affect children: picornaviruses (responsible for illnesses like meningo-encephalitis and sepsis), cytomegalovirus (which can cause severe disabilities in children born with this virus) and HIV. The human-derived organoids or multi-organ systems allow for detailed mechanistic analysis of the disease and possible treatments that can be brought back to the clinic.
Click on the link in the video to watch more or read the interview with Dasja here (https://vu.nl/en/research/more-about/using-human-organoid-technology-to-treat-viral-infections-in-children).

Innovation examples
HealthInnovationIn vitro
Tumor-on-chips to study delivery of protein therapeutics
Valentina is a PhD candidate at the Department of Biochemistry at Radboudumc. Her research focuses on developing and applying organ-on-chip technologies, such as tumor-on-a-chip systems, to study the tissue-specific and cytosolic delivery of protein therapeutics. Valentina's research has also aimed at bridging the gap between engineers and biologists, promoting the use of microfluidic organ-on-chip technologies to answer more relevant biological questions. One example of this is the development of a mathematical model that could be applied to study drug delivery and diffusion in a tumor-on-a-chip system and to extrapolate possible outcomes of the delivery of therapeutic proteins to tumors in the human body. Another collaboration led to the development of a tumor-on-a-chip where hypoxic conditions can be replicated and investigated, and where the targeting of specific hypoxia markers in tumor cells can be investigated.

Innovation examples
ToxicologyInnovationIn vitro
Stem cell differentiation assays for animal-free developmental neurotoxicity assessment
Victoria de Leeuw was a PhD candidate in the research group of prof. dr. Aldert Piersma at the RIVM and Institute for Risk Assessment Sciences at Utrecht University. Piersma's lab studies the effects of compounds on development of the embryo during pregnancy with, among other techniques, stem cell cultures. The project of Victoria was aimed to differentiate embryonic stem cells of mouse and human origin into neuronal and glial cells, which could mimic parts of differentiation as seen during embryonic brain development. These models were able to show some of the known toxic mechanisms induced by these compounds, congruent with what they we hypothesised to mimic. This provides mechanistic information into how chemical compounds can be toxic to brain development. Therefore, these two stem cell assays make a useful contribution to the animal-free assessment of developmental neurotoxicity potential of compounds.
Victoria is nominated for the Hugo van Poelgeest prize 2022 for excellent research to replace animal testing.