Popular

Innovation examples
HealthIn vitroOrgan-on-Chip
An iPSC-derived blood-brain barrier to model neurodegeneration
The blood-brain barrier is a layer of cells that protects our brain from harmful compounds. However, due to this tight barrier, many drugs to treat neurological diseases cannot enter the brain either.
There are currently no good models to test these types of drugs. Henrique Nogueira Pinto is a PhD candidate at the Vrije Universiteit in Amsterdam. He is developing a blood-brain barrier model coupled to mini-brains. With this model, he aims to more reliably test how drugs can be transported over the blood-brain barrier and what their effect on the brain is.
Click on the info button for the full version of the video. Click here (https://fluidsbarrierscns.biomedcentral.com/articles/10.1186/s12987-022-00316-0#Sec3) for a review of the current status of in vitro models for the blood-brain barrier.

Innovation examples
HealthToxicology
Zebrafish in toxicity testing
Zebrafish are increasingly recognised as a useful model for toxicity testing of chemical substances. Testing strategies are becoming more based on mechanisms of toxicity structured in adverse outcome pathways describing the chain of events leading to toxicity or disease. Using a battery of dedicated in vitro and in silico assays, insight can be gained in how exposure leads to disease. For certain diseases it is known that toxicity relies on the interaction between different organs and cell types, which requires research on whole organisms in addition to simple in vitro models. The zebrafish is considered a valuable whole organism model in a mechanism-based testing strategy. At RIVM, the zebrafish embryo model is used for testing the effect of chemical substances on several adverse outcomes and diseases.
For more information see: https://ehp.niehs.nih.gov/doi/10.1289/EHP9888; https://doi.org/10.3390/ijerph18136717; www.linkedin.com/in/harm-heusinkveld

Projects and initiatives
HealthInnovationIn vitro
VitalTissue: scientific research can be more human(e)
The goal of VitalTissue is to facilitate the availability of vital human residual tissue for all researchers in the Netherlands. This video shows how VitalTissue works. From a request from a researcher, the donation of the residual tissue by the patient and the transport to the lab. This process is the result of a feasibility study conducted with many stakeholders. The national tissue bank ETB-BISLIFE will implement VitalTissue in practice.

Innovation examples
HealthToxicologyIn silico
Predictive computer models for protein binding
In this video Linde Schoenmaker (Leiden University) explains how she and her colleagues are making computer models to predict the safety of new chemicals within the VHP4Safety project.

Expert interviews
HealthToxicologyIn vitro
Erwin Roggen, ToxGen Solutions: Applying animal free testing approaches
Erwin Roggen explains his role as pioneer in the development of technologies for animal-free application. His product, ToxGenSolutions, provides test methods required for modern testing and assessment of compounds and products. It builds on a virtual generic platform of leading test and technology developers providing novel technologies addressing key events in adverse outcome pathways.

Expert interviews
HealthToxicology
Kirsten Baken, VITO: Human biomonitoring
Kirsten Baken discusses biomonitoring as part of the HBM4EU project (https://www.hbm4eu.eu). Human biomonitoring is an important component for a new way of doing risk assessment, based on human data. Human-based NAMs can also be informative for human biomonitoring. An example of the use of human biomarkers can be found here (https://www.sciencedirect.com/science/article/pii/S0013935119302658).

Innovation examples
ToxicologyIn vitroOrgan-on-Chip
Cartilage-on-a-chip for studying joint degenerative diseases
Carlo Alberto Paggi is currently a PhD candidate at the University of Twente in the research group of Prof. Marcel Karperien and Prof. Séverine Le Gac. Karperien’s lab focus on the biological aspects of osteoarthritic research while Le Gac’s specialize in organ-on-chip development. The project of Carlo Alberto is developing a joint-on-chip platform to create a reliable in vitro model to study disease progression in osteo- or rheumatoid arthritis. The model combines different organ-on-chips aimed at replicating each a tissue around the joint such as cartilage, bone and ligaments. This new technology focuses on better reproducing human models and at substituting the use of animal models for drug research. If you want to know something more about the project and the groups, you can follow the link in the video.
Carlo Paggi was nominated for the Hugo van Poelgeest prize for his research on a cartilage-on-a-chip model to study joint degenerative diseases
Karperien’s lab of Developmental Bioengineering: https://www.utwente.nl/en/tnw/dbe/
Le Gac’s lab of Applied Microfluidics for BioEngineering Research: http://www.severinelegac.com/
Linkedin: https://www.linkedin.com/in/carlo-alberto-paggi-76500b135/
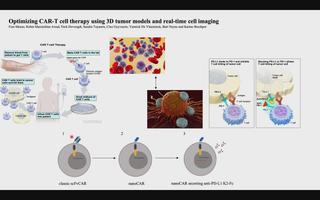
Meetings & conferences
HealthIn vitroAdvanced
3D tumor models for CAR-T-cell therapy optimization
Chimeric antigen receptor (CAR) T-cell therapy accounts for one of the most promising therapeutic advances in cancer immunotherapy. In this form of adoptive cell transfer, T-cells of a patient are engineered to express so-called ‘CARs’, in which the antigen-recognition capacity of antibodies is combined with T-cell activating domains. So far, CAR-T-cell therapy obtained its most impressive results in hematological malignancies resulting in the approval of five CAR-T cell products by the FDA for hematologic indications. However, CAR-T-cell therapy has not mirrored its success in solid tumors. The poor efficacy of CAR-T-cell therapy in solid tumors has, in part, been attributed to the lack of understanding in how CAR-T-cells function in a solid tumor microenvironment. Classical validation methods rely on the use of specificity and functionality assays in 2D models against adherent target cells or target cells in suspension. Yet, by using these models, observations made in vitro may differ greatly to an in vivo situation where tumors are engrafted in 3D structures. We developed a more relevant and translational 3D tumor model using eGFP+ target cells. This allows us to couple 3D tumor cell killing by CAR-T-cells to live-cell imaging, providing an efficient quantification of target cell death. As proof- of-concept, we used a 3D model of eGFP+ glioblastoma cells and CAR-T-cells targeting a pan-cancer antigen. This 3D glioblastoma model allowed us to show that classical scFv-based CAR-T-cell therapy of glioblastoma cells can be improved by nanoCAR-T-cells. Furthermore, combining nanoCAR-T-cell therapy with a genetic approach of nanobody-based anti-PD-L1 immune checkpoint blockade further increased the cytotoxicity of the nanoCAR-T-cell therapy.

Projects and initiatives
HealthInnovationPolicyBeginner
We all want a safer world for humanity, animals and the environment: Transition Animal-free Innovation
Why is the transition to animal-free research so important? What are animal-free models? How does TPI (Transition Animal-Free Innovation) encourage their development and use? And who are we working with to make this happen? We explain this in our animation.
More and more animal-free tests and research methods are becoming available, but not all research questions or safety tests can be answered in this way yet. In addition, the validation, qualification and acceptance of non-animal innovations still lags behind. Therefore, the Dutch Ministry of Agriculture, Nature and Food Quality (LNV) stimulates the development and application of animal-free innovations. This is done with the partner programme Transition Animal-free Innovation (TPI).

Projects and initiatives
HealthToxicologyInnovationIn vitro
Cells4Thought: using iPSCs for neurodevelopmental health
The prevalence of neurodevelopmental disorders (NDDs), including cognitive impairments, is increasing worldwide with great impact on daily life quality. There is evidence that exposure to chemicals may contribute to the incidence of NDD. However, a causal link is lacking. Towards this goal, a human-relevant in vitro model system mimicking parts of brain development, such as neuronal network functioning, could be used for mechanistic research on how gene-environment interactions contribute to the development of NDD. This is going to be studied in the project Cells4Thought, using induced pluripotent stem cells form different individuals to study the effect of chemicals on neuronal differentiation.

Innovation examples
HealthIn vitro
Organoids for studying (personalised) antiviral treatments
Giulia is a scientist in clinical virology with a PhD from OrganoVIR Labs at Amsterdam UMC. Her research aims to improve antiviral testing using human organoids—tiny, lab-grown tissues that mimic real human organs. The COVID-19 pandemic highlighted the urgent need for effective antiviral treatments, as traditional pre-clinical testing on animal models has only a 5% success rate in clinical trials. By utilising human organoids, Giulia enhances the accuracy of antiviral research. She specializes in infecting organoids from the airway, gut, and brain with various patient-derived viruses, allowing for more realistic modelling of viral infections. Her work also sets the stage for personalised medicine in the context of viral infections. By isolating viruses and stem cells from patients suffering from severe infections, she can test tailored treatments that are more likely to succeed. With this, she aims to revolutionise antiviral testing and improve treatment outcomes for patients.
Click on the info button for the full version of the video.

Projects and initiatives
HealthIn vitroOrgan-on-Chip
NXTGEN Hightech Biomed
The Netherlands has strong academic knowledge in areas like Lab-on-Chip, Organ-on-Chip, artificial organs, and cell production technology. However, turning this knowledge into actual products is challenging due to the need for collaboration between different technological and biological specialists. The NXTGEN Hightech program (https://nxtgenhightech.nl/en/biomed/) addresses this by creating a collaborative environment where companies from various fields work together. This approach aims to transform academic insights into innovative products, benefiting both the industry and society.