Innovations

Innovation examples
HealthToxicologyIn silico
AI agents for safer science: How AI is Changing Chemical Risk Assessment
This video introduces a novel approach to chemical safety, where intelligent digital agents guided by large language models support scientists in making faster, more transparent decisions. By automating complex workflows and integrating tools like the OECD QSAR Toolbox, these agentic systems help prioritise research, reduce reliance on animal testing, and pave the way for safer, more sustainable innovation.

Innovation examples
ToxicologyPolicy
User Research in developing the virtual human platform
Digital tools can support the phasing out of animal-based tests and data in chemical risk assessment. This is one of the core promises of the Virtual Human Platform. The potential contribution of digitalization is linked to the acceptance and adoption of tools, methods, and data by stakeholders in several societal sectors. To facilitate the integration of stakeholders in the configuration of digital tools, Dr. Isaac Ortega Alvarado and colleagues gather insights from risk assessors in their role as users. Risk assessors are the ones who actualize chemical risk assessment and its standards through their practices. With this perspective, this research contributes to understanding the development and implementation of digital tools as embedded in social processes of construction and reception.

Innovation examples
HealthToxicologyIn silico
Predictive computer models for protein binding
In this video Linde Schoenmaker (Leiden University) explains how she and her colleagues are making computer models to predict the safety of new chemicals within the VHP4Safety project.

Innovation examples
HealthToxicologyIn vitro
Thyroid Hormone & Brain Development: animal-free models for human safety assessment
The environment can have a significant impact on a child's health even before birth. Brain development begins in the first trimester and continues until the age of 25, with thyroid hormone playing a critical role. During early pregnancy, the fetus depends on the mother's thyroid hormone, and a disruption in the thyroid hormone balance can lead to cognitive and motor impairments in the child. As part of the VHP4Safety project, we are developing in vitro tests to measure the developmental neurotoxic effects caused by disturbances thyroid hormone concentrations. Current testing guidelines do not always include testing for neurodevelopmental effects, highlighting the need for new non-animal methods. At the Erasmus Medical Center, human cell lines representing brain cell types are cultured to study the effect of chemicals on the thyroid hormone balance. RIVM uses human stem cells to create neuron-astrocyte networks that mimic brain development. By combining these different assays and models, we are creating a comprehensive human-based testing strategy to assess developmental neurotoxicity. These advances are a critical step toward eliminating animal testing while protecting the health and environment of future generations.

Innovation examples
HealthIn vitro
Cultured human skin for burn research
Burns are often accompanied by a dysregulated immune response, which can lead to systemic inflammation, impaired immunity, and excessive scarring. A deeper understanding of the mechanisms behind burns—where wound healing and inflammatory reactions are severely disrupted—holds the key to improving patient outcomes. Patrick Mulder, a postdoctoral researcher at the Burn Research Lab in Beverwijk, the Netherlands, works with his colleagues to develop animal-free skin models based on human cells and patient-derived tissues. Using these innovative, human-relevant models, he aims to provide greater insight into the body’s response to burns and studies the effects of existing and new treatments on wound healing.
Click on the info button for the full version of the video.

Innovation examples
HealthIn vitroOrgan-on-Chip
An iPSC-derived blood-brain barrier to model neurodegeneration
The blood-brain barrier is a layer of cells that protects our brain from harmful compounds. However, due to this tight barrier, many drugs to treat neurological diseases cannot enter the brain either.
There are currently no good models to test these types of drugs. Henrique Nogueira Pinto is a PhD candidate at the Vrije Universiteit in Amsterdam. He is developing a blood-brain barrier model coupled to mini-brains. With this model, he aims to more reliably test how drugs can be transported over the blood-brain barrier and what their effect on the brain is.
Click on the info button for the full version of the video. Click here (https://fluidsbarrierscns.biomedcentral.com/articles/10.1186/s12987-022-00316-0#Sec3) for a review of the current status of in vitro models for the blood-brain barrier.

Innovation examples
HealthIn vitro
Organoids for studying (personalised) antiviral treatments
Giulia is a scientist in clinical virology with a PhD from OrganoVIR Labs at Amsterdam UMC. Her research aims to improve antiviral testing using human organoids—tiny, lab-grown tissues that mimic real human organs. The COVID-19 pandemic highlighted the urgent need for effective antiviral treatments, as traditional pre-clinical testing on animal models has only a 5% success rate in clinical trials. By utilising human organoids, Giulia enhances the accuracy of antiviral research. She specializes in infecting organoids from the airway, gut, and brain with various patient-derived viruses, allowing for more realistic modelling of viral infections. Her work also sets the stage for personalised medicine in the context of viral infections. By isolating viruses and stem cells from patients suffering from severe infections, she can test tailored treatments that are more likely to succeed. With this, she aims to revolutionise antiviral testing and improve treatment outcomes for patients.
Click on the info button for the full version of the video.

Innovation examples
ToxicologyIn vitro
Combatting the worlds deadliest infections using groundbreaking human-mimetic tools
Combatting the worlds deadliest infections using groundbreaking human-mimetic tools.
Zika, dengue & other viruses are typically tested in monkeys & mice. But is using animals really the most effective way? Find out what Dr. David Pamies of the @jhucaat - along with his colleagues at @JohnsHopkins - are doing to upend the status quo.
More information on:
https://www.eurekalert.org/pub_releases/2019-06/hsi-ctw061319.php
and
https://www.frontiersin.org/articles/10.3389/fcimb.2019.00223/
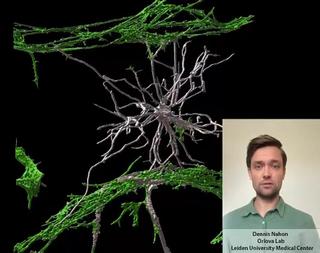
Innovation examples
HealthIn vitroOrgan-on-Chip
Stem cell derived Vessels-on-Chip to study brain disorders
Dennis Nahon is a PhD candidate in the Department of Anatomy and Embryology at the Leiden University Medical Center. In his research, under supervision of Dr. Valeria Orlova (https://www.orlovalab.com/) and Prof. Dr. Christine Mummery, he aims to mimic a blood vessel in the brain by combining different stem cell derived cell types, in a 3D Vessel-on-Chip model. Here, an example of these in vitro blood vessels is shown in which certain brain cells known as astrocytes (in white) interact with the blood vessels (in red). This model paves the way for investigating brain vessels outside the human body, while reducing the need for animal models.

Innovation examples
In vitroOrgan-on-Chip
From 2D hiPSC culture to developing a 3D vessel-on-chip
Theano Tsikari is a 2nd year PhD student at the Orlova group at LUMC. As part of the LymphChip consortium, her project focuses on the development of immunocompetent organ-on-chip models of the cardiovascular system, and especially the integration of tissue-resident macrophages and lymphatic vasculature using human induced pluripotent stem cells. In this video, you can follow her as she presents you the backbone of her project, a 3D hiPSC-derived vessel-on-chip model, that has been previously developed in the Orlova group and can be employed for the generation of advanced in vitro models of vascular diseases.

Innovation examples
In vitroOrgan-on-Chip
Unified organoid system for modeling heart and kidney interaction on-a-chip
Beatrice Gabbin is a PhD candidate at the Anatomy and Embryology Department of the Leiden University Medical Center. Her project is shared with the Nephrology Department and focusses on the study of the cardiorenal axis in vitro. Both heart and kidneys have vital functions in the human body and reciprocally influence each other’s behavior: pathological changes in one can damage the other. There are already multiple independent in vitro (human) models of heart and kidney, but none have so far captured their dynamic crosstalk. The aim of the project is therefore to develop a microfluidic system which can be used to study heart and kidney interaction in vitro. For this purpose, cardiac microtissues and kidney organoids derived from human induced pluripotent stem cells are generated and loaded onto a 3D perfusion chip for their dynamic co-culture. This system enables the study the cardiac and kidney interaction with a high level of control. The validation of a unified organoid system will enable the investigation of diseases involving the two organs and their potential treatments. Read more via the link in the video and https://doi.org/10.1016/j.mtbio.2023.100818.

Innovation examples
HealthIn vitroOrgan-on-Chip
Modelling COVID-19-induced thrombosis using blood-perfused Vessels-on-Chips
A subset of hospitalized COVID-19 patients develops severe symptoms like microthrombosis and multiple organ-failure, worsening survival rates. The most inner layer of cells of a blood vessel, the endothelial cells, play a central role in the development of these complications. Their dysfunction can be replicated in advanced cell culture models like our blood-perfused Vessel-on-Chip to further understand disease mechanisms. In this short highlight, Huub Weener from the University of Twente shows how the technique works and what these models contribute to our knowledge of COVID-19.